Our work on horseshoe crabs spans from molecular studies of second messengers to field studies of mating behavior. Prior to 2000, most of my research, in collaboration with numerous colleagues and students was focused on Limulus ventilation, gill gleaning, cardiac physiology, CNS central pattern generators, cardiac ganglion neurophysiology and modulation of neural networks and muscles by neuropeptides and biogenic amines. In the past 25 years we've been paying more attention to the behavior of horseshoe crabs in the field, with a focus on biological rhythms, seasonal movements, and spawning. We have also used our expertise in these areas to investigate the impacts that bleeding horseshoe crabs to obtain Limulus amoebocyte lysate (LAL) has on their overall health and behavior. For additional details about any of this work, download some of our papers listed in the Publications section of this website.

The studies of Barlow, Chamberlain, Kass, Dodge and their colleagues have clearly demonstrated the presence of a circadian clock in the brain of horseshoe crabs that controls visual sensitivity. This clock communicates with the eyes via efferent nerves. When these nerves are active, at night, they release a substance on the eyes which makes them much more sensitive to light. They estimate this increase in sensitivity is on the order of 100,000 fold or more. Recently, we devised a method for recording the locomotory activity of horseshoe crabs in the laboratory, using modified running wheels (see Figure 1 below). We also developed a technique that allows us to simultaneously record changes in the sensitivity of one of the Limulus lateral eyes AND locomotion (see Figure 2 below). These two novel methods have allowed us to test the hypothesis that both eye sensitivity rhythms and activity rhythms are controlled by the same clock.
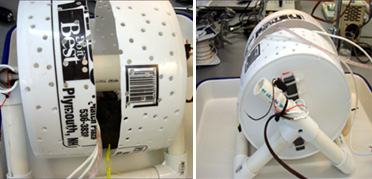
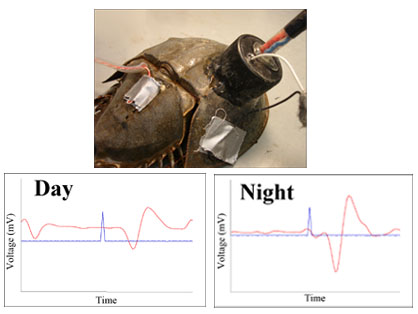
Our preliminary results indicate that eye sensitivity rhythms are strickly circadian and readily entrain to imposed light:dark cycles, while activity rhythms are quite flexible, and can be either tidal, diurnal or nocturnal. Finally, we have determined that tidal rhythms of activity can be entrained by small, imposed, tidal cycles. How horseshoe crabs detect these changes in water depth is an ongoing area of investigation. Figure 3 shows data from an animal who, for a few days, was nocturnal and thus its activity was correlated with its eye sensitivity rhythm. Figure 4 is more typical, with a tidal rhythm of activity, but a circadian rhythm of visual sensitivity entrained to the imposed L:D cycle.


Since 2005 we have been tracking the movements of horseshoe crabs in the Great Bay estuary, NH using a variety of ultrasonic telemetry techniques. We investigate small scale movements using a fixed array system, involving three buoys that communicate with a shore station (Vemco VRAP system). Information about large scale movements over periods of time ranging from days to months is obtained using both manual tracking methods and VR2 "listening stations".
We seek to answer the following questions during these investigations:
1. Do horseshoe crabs express the same types of circadian and tidal rhythms observed in the laboratory in the field? If so, do they express different rhythms at different times of year? If not, why not?
2. What conditions in the estuary appear to have the greatest impact on horseshoe crab movements? Tides? Salinity? Thermal gradients?
3. Do horseshoe crabs exhibit a predictable pattern of seasonal movements, moving into the estuary in the spring, and down toward the coast in the summer/fall?
What have we found?
Mating Surveys
We were curious if more horseshoe crabs mate during daytime high tides than nighttime high tides so we did surveys during both tides on the same day, throughout the mating season. Our data indicates that, while there is considerable variability, about the same number of animals mate during the day as during the night. Furthermore, contrary to some previous studies, conducted primarily in the southern end of their range, where the full and new moon tides are much higher than during the rest of the month, we found very little correlation between moon phase and spawning. Our data suggests that the first big peak in spawning is correlated with rapidly increasing water temperatures and our telemetry studies demonstrated that horseshoe crabs begin their movements up into Great Bay in the spring when the water temperature rises about 12 degrees C.
Seasonal Movements
The ultrasonic tags we use last for about a year. Therefore, we are able to monitor the seasonal movements of horseshoe crabs. They in the spring they move into shallow water at least a month before they mate. The animals we tracked in 2005-6 then moved up into the estuary to mate before moving back down the estuary in the summer. They then overwintered in fairly deep water, but not more than 3-4 kms from where they mate. No horseshoe crabs left the estuary during any of our studies, suggesting there is a Great Bay subpopulation. This is worth investigating further using some genomics approaches.
Daily Movements
Both our laboratory studies and field studies indicate that horseshoe crabs are most active during high tides and this is driven, in part, by their endogenous circatidal clock. They are also sensitive to changes in water depth, which helps entrain their clock to the natural tidal cycles. In any given day, they will move into shallow areas, with soft sediment and dig for prey items to feed upon, before returning to deeper water where they bury and wait out the low tide periods.
The following publications provide further details about horseshoe crab movements in their natural habitat:
Chabot, C.C., J. Kent and W.H. Watson III. 2004. Circatidal and circadian rhythms of locomotion in Limulus polyphemus. Biol. Bull. 207: 72-75. https://doi.org/10.2307/1543630
Chabot, C.C., S.H. Betournay, N.R. Braley and W.H. Watson III. 2007. Endogenous rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. J. Exp. Marine Biol. and Ecology 345: 79-89.
Chabot, C.C., S. Skinner and W.H. Watson III. 2008. Artificial tides synchronize circatidal rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. Biol. Bull. 215: 34-45.
Chabot CC, Yelle JF, O’Donnell CB, Watson WH. 2010. The effects of water pressure, temperature and current cycles on circatidal rhythms expressed by the American horseshoe crab, Limulus polyphemus. Mar. Fresh. Behav. Physiol. 44: 43-60.
Schaller, S.Y., Watson WH, Chabot CC, 2010. Seasonal movements of American horseshoe crabs, Limulus polyphemus, in the Great Bay Estuary, New Hampshire (USA). Curr. Zool. 56(5): 587-598.
Watson WH, Chabot CC, 2010. High resolution tracking of adult horseshoe crabs (Limulus polyphemus) in a New Hampshire estuary using fixed array ultrasonic telemetry. Curr. Zool. 56(5): 599-610.
Chabot CC, Watson WH III, 2010. Circatidal rhythms of locomotion in the American horseshoe crab, Limulus polyphemus: Underlying mechanisms and cues that influence them. Current Zoology 56(5): 499-517.
Chabot, C.C., N. Ramberg-Pihl*, W.H Watson. 2016. Circalunidian clocks control tidal rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. Mar. Fresh Behav. Physiol. 49: 75-91. doi.org/10.1080/10236244.2015.1127679
Cheng, H, CC Chabot and WH Watson III. 2016. Influence of environmental factors on spawning of the American horseshoe crab (Limulus polyphemus) in the Great Bay Estuary, USA. Estuaries and Coasts 39(4): 1142-1153. doi.org/10.1007/s12237-015-0044-2.
Watson, WH III, SK Johnson, CD Whitworth and CC Chabot. 2016. Rhythms of locomotion and seasonal changes in activity expressed by horseshoe crabs in their natural habitat. Mar. Ecol. Prog. Ser. 542: 109-121. doi:10.3354/meps11556.
Chabot, C.C., N. Ramberg-Pihl*, W.H Watson. 2016. Circalunidian clocks control tidal rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. Mar. Fresh Behav. Physiol. 49: 75-91. doi.org/10.1080/10236244.2015.1127679
Chesmore, KN, WH Watson III and CC Chabot. 2016. Identification of putative circadian clock genes in the American horseshoe crab, Limulus Polyphemus. Comp. Biochem. Physiol. (D) 19: 45-62. doi.org/10.1016/j.cbd.2016.06.001
Anderson, R.L., W.H. Watson III and C.C. Chabot. 2017. Local tidal regime dictates plasticity of expression of locomotor activity rhythms of American horseshoe crabs, Limulus Polyphemus. Marine Biol. 164: 63-75. doi.org/10.1007/s00227-017-3098-9
Dubofsky, B.A., W.H. Watson, and C.C. Chabot. 2017. Entrainment of Juvenile Horseshoe Crab Activity to Artificial Tides. Mar Fresh Behav Physiol. 50:125-140. doi.org/10.1080/10236244.2017.1344525.
Thomas, T.N., W.H. Watson III and C.C. Chabot. 2020. The relative influence of nature vs. nurture on the expression of circatidal rhythms in the American horseshoe crab Limulus polyphemus. MEPS 649: 83-96. doi.org/10.3354/meps13454.
Cheng, H., C.C. Chabot and W.H. Watson III. 2021. The distribution of juvenile American horseshoe crabs (Limulus polyphemus) in the Great Bay Estuary, New Hampshire U.S.A. Mar. Ecol. Prog. Series. 662: 199-203. doi.org/10.3354/meps13611
Cheng, H., V. Vaattovaara, M. Connelly, B. Looney, C. C. Chabot and W. H. Watson III. 2022. Temperature and salinity preference of adult American horseshoe crabs (Limulus polyphemus). In: J. T. Tanacredi et al. (eds.), International Horseshoe Crab Conservation and Research Efforts: 2007-2020, doi.org/10.1007/978-3-030-82315-3_33. Pp. 581-598.
Almost a million horseshoe crabs are bled each year to obtain LAL, which is used extensively to test products, such as vaccines, that are used in hospitals throughout the world. During this process ~30% of their blood is removed. Approximately 15-30% of the animals that are bled die as a result of the procedure and the others are released. We have been trying to determine if there are sublethal impacts of the process on those animals that are released and we have also been trying to figure out ways to make the procedure less stressful and thus more sustainable.
We found that the combination of stressor involved in the whole process, including warm temperatures and being out of water for long periods of time, is much worse than just the loss of blood itself. We also found that loss of the respiratory pigment, hemocyanin, was likely causing most of the health issues, including mortality and reduced overall activity. This loss of activity manifested itself in the field in terms of mating behavior. We found that females attempted to spawn 50% fewer times if they had been bled.
In order to alleviate some of the repercussions of bleeding, we have been attempting to identify a food supplement that can help horseshoe crabs recover their hemocyanin levels more rapidly after being bled. Our preliminary trials have been successful and we hope the industry to attempt to carry forward with this approach.
The following publications provide further details about our work in this area:
Anderson, R.L., W.H. Watson III and C.C. Chabot. 2013. Sublethal behavioral and physiological effects of the biomedical bleeding process on the American horseshoe crab, Limulus polyphemus. Biol. Bull. 225: 137-151. doi.org/10.1086/BBLv225n3p137
Owings, M., C. C. Chabot and W. H. Watson III. 2019. Effects of the biomedical bleeding process on the behavior of the American horseshoe crab, Limulus polyphemus, in its natural habitat. Biol. Bull. 236(3): 207-223. doi.org/10.1086/702917
Owings, M., C.C. Chabot and W.H. Watson III. 2020. Effects of the biomedical bleeding process on the behavior and hemocyanin levels of the American horseshoe crab (Limulus polyphemus). Fish. Bull. 118: 225-239. doi: 10.7755/FB.118.3.2
Click here for additional information about horseshoe crabs.